AFP
Feb 8, 2008
US drug watchdog warns of Botox hazards
AFP
Feb 8, 2008
WASHINGTON, Feb 8, 2008 (AFP) - The US Food and Drug Administration (FDA) warned Friday February 8th that using botulinum toxins, such as the popular cosmetic treatment Botox, can have serious side-effects including death, but stopped short of banning them.
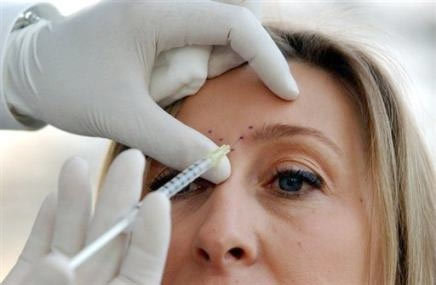 A injection of Botox - Photo : AFP |
"The agency announced today that we've become aware of a number of reports of serious adverse reactions from botulinum toxins," including breathing difficulties and death, Dr Russell Katz, the head of the FDA's division of neurology products, said.
"These are reactions distant from where the product was injected," he told a telephone news conference.
In a statement issued before the news conference, the FDA said the adverse reactions were "suggestive of botulism, which occurs when botulinum toxin spreads in the body beyond the site where it was injected.
"The most serious cases had outcomes that included hospitalization and death," it added.
Botulinum toxins are approved by the FDA to treat a variety of symptoms including spasms of the eyelids or neck, the easing of facial lines or excessive sweating.
Most of the severe reactions and deaths seen in the review occurred in children who were given botulinum toxins in the legs to control spasticity related to cerebral palsy -- one of many indications that physicians prescribe botulinum toxins for, even though it is "off-label" or not approved by the FDA, Katz said.
"We believe the toxin spread from there to affect breathing," he said.
Katz stressed that no patients who had used Botox for cosmetic purposes were among the fatalities, but urged vigilance.
"If someone uses Botox cosmetically and develops symptoms that might be related to this distant spread, physicians and patients should take it seriously," he said.
He insisted that the FDA warning had not been prompted by a call from a consumer rights group two weeks ago, urging that Botox, the botulinum toxin used by millions around the world to iron out wrinkles, carry more stringent health warnings.
Advocacy group Public Citizen, founded by former presidential candidate Ralph Nader, called last month for the FDA to "immediately increase its warnings... about the use of botulinum toxin" because of "serious adverse reactions, including deaths, linked to the drug."
The group said that between November 1997 and December 2006 there were 16 deaths among the 658 reported cases of people "suffering adverse effects from injections of botulinum toxin."
Katz said data studied by the FDA had shown "a relative handful" of cases of severe adverse reactions related to the distant spread of the toxin, but refused to give a precise figure, saying the review was in the early stages.
Botox, Botox Cosmetic and Myobloc warn on their labeling of the possibility of adverse reactions near the site of the injection for each product's approved uses, according to the FDA, and of "the rare potential of distant side effects" including severe difficulty swallowing and difficulty breathing when the products are used on patients with neuromuscular disorders, said Katz.
The FDA review appears to indicate that distant side effects can occur in a broader population, said Katz.
But the agency insisted it has "not concluded there is a causal relationship between the drug products and the emerging safety issue" and did not "advise health care professionals to discontinue prescribing these products."
"FDA is considering, but has not reached a conclusion about whether this information warrants any regulatory action," the agency said.
The botulinum toxin is a natural poison found in decomposing food that is 40 million times more powerful than cyanide.
When injected, tiny doses of the toxin paralyze a muscle and prevent it from contracting for between four and six months -- ideal for temporarily eliminating facial wrinkles but potentially deadly if it affects the wrong muscles.
Copyright © 2024 AFP. All rights reserved. All information displayed in this section (dispatches, photographs, logos) are protected by intellectual property rights owned by Agence France-Presse. As a consequence you may not copy, reproduce, modify, transmit, publish, display or in any way commercially exploit any of the contents of this section without the prior written consent of Agence France-Presses.

























